| Project Leader | Haruo TAKABAYASHI Director of FDD-MB Center, Kanazawa Medical University |
||||
| U R L | http://IDM-World.com/FDD-MB/ | ||||
|
|||||
This system completely eliminates the physical and mental burden of testing on mothers and the risk of miscarriage. Because this system makes it possible to safely obtain fetal DNA information in the early stage of pregnancy, it is also expected to contribute to the development of effective early treatment for various diseases in infants. It has become known that fetal cells are transferred into maternal blood, prompting keen interest in the involvement of fetal cells in female health and the development of safe fetal DNA diagnosis methods. Research groups both at home and abroad have been carrying out trials on the selective collection of fetal cells and performing DNA diagnosis.
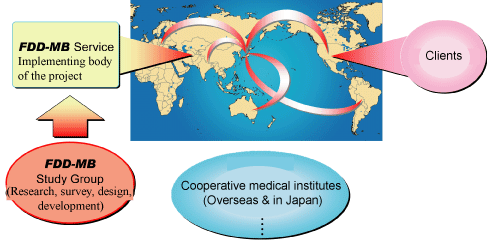
A secure and effective technique for the collection of fetal cells contained in maternal blood (Normally only 1 fetal cell is contained per 1ml of maternal blood.) is essential for safe fetal DNA diagnosis. We focused attention on fetal cells that transfer into maternal blood, established a method of selecting and collecting such fetal cells (nucleated RBC) using unique technologies, and clarified the possibility of DNA analysis using a single cell. Based on the results of our work, we are planning to complete work on the world’s first safe fetal DNA diagnosis system (FDD-MB 3.0), the most advanced system available for practical use. This diagnosis system is being developed through the collaborative efforts of researchers at medical institutes in Ishikawa, Toyama, and other areas in Japan as well as overseas sites.
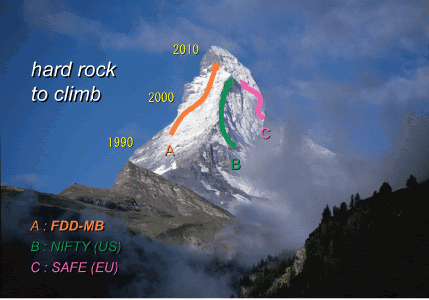
Although in Europe and U.S., large scale clinical studies (NIFTY study by NIH in U.S., etc.) were carried out at considerable cost, development was held up due to limitations in existing method (antigen-antibody reaction). Meanwhile, a safe fetal cell collection and diagnosis system (FDD-MB) utilizing Percoll-micromanipulation technology that we developed has been highly regarded as a stable and useful method, and it is obvious that this Japanese system is the closest to being developed as the world’s first practical/commercialized system.
Finally, the realization of this advanced DNA diagnosis system not only promotes the rescue and early treatment of infants, but it also exerts a pervasive influence on the enhancement of health and the prevention of disease for females nurturing infants. This contributes not only to the reinforcement of medical services in Japan, but also to global innovation and economic influence on medical science and medical care. Currently, prenatal testing is carried out 5 million times annually throughout the world. The commercialization of FDD-MB system, which is a safe prenatal testing method, is predicted to achieve a high share in the global market.
Research and development on this system is expected to result in significant innovation and contributions to perinatal and DNA-related medical care. We are working hard to improve the system for practical use through the clinical application of our elemental technologies to contribute to female healthcare. We also aim to start providing testing services in FY 2010 by advancing the preparation for practical use in cooperation with SC World, Inc. and other affiliated companies as well as through continuing research and development. Furthermore, we are promoting introduction of this diagnosis system and reagent kit to the market.
In the future, we will develop several bases in Japan, including the Ishikawa University Collaborative Incubator and several bases overseas by taking account of geographical conditions and accessibility to lead cluster formation and the establishment of business models at the global level.
The Hokuriku Industrial Advancement Center